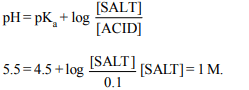1. The pH of a 1 M \[CH_{3}COONa\] solution in water will be nearly
a) 2.4
b) 5.4
c) 7.4
d) 9.4
Explanation:
2. A physician wishes to prepare a buffer solution at
pH = 3.58 that efficiently resists a change in pH yet contains
only small conc. of the buffering agents. Which one of the
following weak acid together with its sodium salt would be
best to use?
a) m-chloro benzoic acid \[\left(pK_{a} = 3.98\right)\]
b) p-chloro cinnamic acid \[\left(pK_{a} = 4.41\right)\]
c) 2,5-dihydroxy benzoic acid \[\left(pK_{a} = 2.97\right) \]
d) Acetoacetic acid \[\left(pK_{a} = 3.58\right) \]
Explanation:
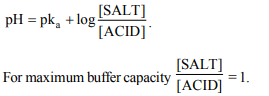
3. Which one is Buffer solution?
a) \[\left[PO_4^{-3} \right]\left[HPO_4^{2-}\right]\]
b) \[\left[PO_4^{3-} \right]\left[H_{2}PO_4^-\right]\]
c) \[\left[HPO_4^{2-} \right]\left[H_{2}PO_4^-\right]\]
d) All of these
Explanation:
4. Which of the following solution cannot act as a buffer?
a) \[NaH_{2}PO_{4}+H_{3}PO_{4}\]
b) \[CH_{3}COOH+CH_{3}COONa\]
c) \[HCl+NH_{4}Cl\]
d) \[H_{3}PO_{4}+NaH_{2}PO_{4}\]
Explanation: HCl + NH4Cl. HCl is strong acid hence not used in buffer
5. A buffer solution of pH 9 can be prepared by mixing
a) \[CH_{3}COONa\] and \[CH_{3}COOH\]
b) NaCl and NaOH
c) \[NH_{4}Cl\] and \[NH_{4}OH\]
d) \[KH_{2}PO_{4}\] and \[K_{2}HPO_{4}\]
Explanation: The pH of basic buffer is more than 7. NH4Cl and NH4OH combination provides basic buffer
6. Which of the following is the buffer solution of strong acidic
nature?
a) \[HCOOH + HCOO^{-}\]
b) \[CH_{3}COOH+ CH_{3}COO^{-}\]
c) \[H_{2}C_{2}O_{4}+ C_{2}O_4^{2-}\]
d) \[H_{3}BO_{3}+ BO_3^{3-}\]
Explanation: Formic acid is stronger than other acids given.
7. One litre of a buffer solution containing 0.01 M \[NH_{4}Cl\] and
0.1 M \[NH_{4}OH\] having pKb of 5 has pH of
a) 9
b) 10
c) 4
d) 6
Explanation:
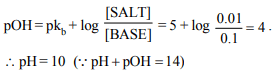
8. A certain buffer solution contains equal concentration of
X– and HX. The Ka for HX is \[10^{-8}\] . The pH of the buffer is
a) 3
b) 8
c) 11
d) 14
Explanation:
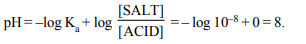
9. In a mixture of a weak acid and its salt, the ratio of the
concentration of acid to salt is increased tenfold. The pH of
the solution
a) decreases by one
b) decreases by one tenth
c) increases by one
d) increases by ten-fold
Explanation:
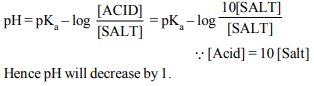
10. How much sodium acetate should be added to 0.1 M solution
of \[CH_{3}COOH\] to give a solution of pH 5.5 \[(pK_{a}\] of \[CH_{3}COOH =4.5)\]
a) 0.1 M
b) 0.2 M
c) 1.0 M
d) 10.0 M
Explanation: