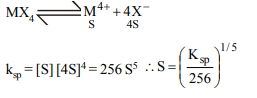1.Identify the correct order of solubility in aqueous medium
a) \[ZnS > Na_{2}S > CuS\]
b) \[ Na_{2}S > CuS>ZnS\]
c) \[ Na_{2}S > ZnS>CuS\]
d) \[ CuS > ZnS>Na_{2}S\]
Explanation: Solubility of alkali metal is maximum among the following. Among ZnS (1.7 × 10-5) & CuS (8 × 10–37) ZnS has higher value of Ksp.
2. Which of these is least likely to act as Lewis base?
a) \[F^{-}\]
b) \[BF_{3}\]
c) \[PF_{3}\]
d) CO
Explanation: BF3 is Lewis acid(e– pair acceptor)
3. 1 M NaCl and 1 M HCl are present in an aqueous solution.
The solution is
a) not a buffer solution with pH < 7
b) not a buffer solution with pH > 7
c) a buffer solution with pH < 7
d) a buffer solution with pH > 7
Explanation: A buffer is a solution of weak acid and its salt with strong base and vice versa. HCl is strong acid and NaCl is its salt with strong base. pH is less than 7 due to HCl
4. Species acting as both Bronsted acid and base is
a) \[\left(HSO_{4}\right)^{-1}\]
b) \[Na_{2}CO_{3}\]
c) \[NH_{3}\]
d) \[OH^{-1}\]
Explanation:
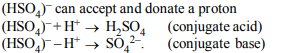
5. Let the solubility of an aqueous solution of \[Mg\left(OH\right)_{2}\]
be x then its \[K_{sp}\] is
a) \[4x^{3}\]
b) \[108x^{5}\]
c) \[27x^{4}\]
d) 9x
Explanation:
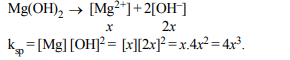
6. The solubility of \[Mg\left(OH\right)_{2}\] is S moles/litre.
The solubility product under the same condition
is
a) \[4S^{3}\]
b) \[3S^{4}\]
c) \[4S^{2}\]
d) \[S^{3}\]
Explanation:
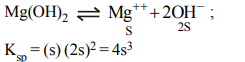
7. Which one of the following statements is not
true?
a) pH + pOH = 14 for all aqueous solutions
b) The pH of \[1 × 10^{-8}\] M HCl is 8
c) 96,500 coulombs of electricity when passed through a
\[CuSO_{4}\] solution deposits 1 gram equivalent of copper at
the cathode
d) The conjugate base of \[H_{2}PO_4^-\] is \[HPO_4^{2-}\]
Explanation: An acidic solution cannot have a pH > 7.
8.The solubility in water of a sparingly soluble salt \[AB_{2}\] is \[1.0 × 10^{-5}mol^{-1}\] . Its solubility product number
will be
a) \[4 × 10^{-10}\]
b) \[1 × 10^{-15}\]
c) \[1 × 10^{-10}\]
d) \[4 × 10^{-15}\]
Explanation:
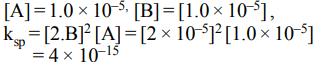
9.The conjugate base of
\[H_{2}PO_4^-\] is
a) \[H_{3}PO_{4}\]
b) \[P_{2}O_{5}\]
c) \[PO_4^{3-}\]
d) \[HPO_4^{2-}\]
Explanation:
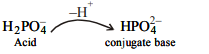
10. The molar solubility (in mol \[L^{-1})\] of a sparingly
soluble salt \[MX_{4}\] is ‘S’. The corresponding solubility product
is \[K_{sp}\] . ‘S’ is given in term of \[K_{sp}\] by the relation :
a) \[S=\left(256K_{sp}\right)^{1/5}\]
b) \[S=\left(128K_{sp}\right)^{1/4}\]
c) \[S=\left(K_{sp}/128\right)^{1/4}\]
d) \[S=\left(K_{sp}/256\right)^{1/5}\]
Explanation: