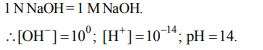1. pH of 10 M solution of HCl is
a) 1
b) 0
c) 2
d) less than 0
Explanation: – log [H+ ] = pH; – log 10 = pH. Hence pH = –1 less than 0
2. It is found that 0.1 M solution of four sodium salts NaA,
NaB, NaC and NaD have the following pH values
7.0, 9.0, 10.0 and 11.0 respectively
a) NaD
b) NaC
c) NaB
d) NaA
Explanation: The pH values indicate that NaD, NaC and NaB are salts of strong base and weak acid. pH of NaA = 7 it is salt of strong acid and strong base
3. A white salt is readily soluble in water and gives a colourless
solution with a pH of about 9. The salt would be
a) \[NH_{4}NO_{3}\]
b) \[CH_{3}COONa\]
c) \[CH_{4}COONH_{4}\]
d) \[CaCO_{3}\]
Explanation: Salt must be of strong base and weak acid as pH = 9, hence the salt CH3COONa
4. The value of ionic product of water at 393 K is
a) less than \[1 × 10^{-14}\]
b) greater than \[1 × 10^{-14}\]
c) equal than \[1 × 10^{-14}\]
d) equal than \[1 × 10^{-7}\]
Explanation: Kw increases with temperature.
5. Which has the highest pH?
a) \[CH_{3}COOK\]
b) \[Na_{2}CO_{3}\]
c) \[NH_{4}Cl\]
d) \[NaNO_{3}\]
Explanation: The higher the pH more, the basic character, pH of Na2CO3 > CH3COOK.
6. A solution of \[MgCl_{2}\] in water has pH
a) < 7
b) > 7
c) 7
d) 14.2
Explanation: MgCl2 gives acidic solution hence pH < 7
7. A solution of an acid has pH = 4.70. Find out the number of \[OH^{-}\] ions \[\left(pK_{W}=14\right)\]
a) \[5 × 10^{-10}\]
b) \[4 × 10^{-10}\]
c) \[2 × 10^{-5}\]
d) \[9 × 10^{-4}\]
Explanation:
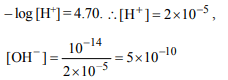
8. The pH of a solution whose \[\left[H^{+}\right]\] is \[3.0 × 10^{-4}M\] is
a) 4.45
b) 3.75
c) 4.36
d) 3.523
Explanation:
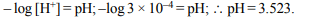
9. pH of 4.0 gm/litre NaOH solution is
a) 13
b) 11
c) 13.5
d) 12
Explanation:
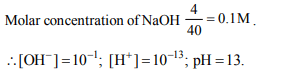
10. Highest pH (14) is given by
a) 0.1 M \[H_{2}SO_{4}\]
b) 0.1 M NaOH
c) 1 N NaOH
d) 1 N HCl
Explanation: