1. Which of the following is strongest Lewis base?
a) \[CH_3^-\]
b) \[NH_2^-\]
c) \[OH^-\]
d) \[F^-\]
Explanation: \[CH_3^-\]
2. Aluminium chloride is
a) Bronsted Lowry acid
b) Arrhenius acid
c) Lewis acid
d) Lewis base
Explanation: AlCl3 electron deficient hence Lewis acid
3. \[BF_{3}\] is an acid according to
a) Arrhenius concept
b) Bronsted-Lowry concept
c) Lewis Concept
d) Both (b) and (c)
Explanation: Lewis concept
4. Which of the following is not a Lewis acid?
a) CO
b) \[SiCl_{4}\]
c) \[SO_{3}\]
d) \[Zn^{2+}\]
Explanation: CO cannot accept electrons. Hence it is not Lewis acid
5. Ammonium ion is
a) a conjugate acid
b) a conjugate base
c) both an acid and a base
d) neither an acid nor a base
Explanation:
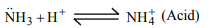
6. Among the following, the weakest base is
a) \[H^{-}\]
b) \[CH_3^-\]
c) \[CH_{3}O^{-}\]
d) \[Cl^{-}\]
Explanation: HCl is strongest hence Cl– weakest.
7.In the equation \[I_2 + I^{-} \rightarrow I_{3}^{-}\] , which is the Lewis base?
a) \[I_2\]
b) \[I^{-}\]
c) \[I_{3}^{-}\]
d) None of these
Explanation: I– is electron donor. Hence it is Lewis base
8.Which one of the following compounds is not a protonic
acid
a) \[PO\left(OH\right)_3\]
b) \[SO\left(OH\right)_2\]
c) \[SO_2\left(OH\right)_2\]
d) \[B\left(OH\right)_3\]
Explanation: The acids are H3 PO4 (orthophosphoric acid), H2 SO3 (Sulphurous acid) and H2SO4 (Sulphuric acid) are protonic acid whereas Orthoboric acid is not protonic acid.
9. Which equilibrium can be described as an acid-base reaction
using the Lewis acid-base definition but not using the
Bronsted-Lowry definition?
a) \[2NH_3 + H_2SO_4\rightleftharpoons 2NH_4^+ + SO_4^{2-}\]
b) \[NH_3 + CH_3 COOH \rightleftharpoons NH_4^+ + CH_3 COO^-\]
c) \[H_2 O+ CH_3 COOH \rightleftharpoons H_3 O^+ + CH_3 COO^-\]
d) \[\left[Cu\left(H_2 O\right)_4\right]^{2-}+ 4NH_3 \rightleftharpoons \left[Cu\left(NH_3 \right)_4\right]^{2+} + 4H_2O \]
Explanation:

10. Which one of the following is the strongest acid?
a) \[ ClO_3 OH\]
b) \[ClO_2\left( OH\right)\]
c) \[SO\left( OH\right)_2\]
d) \[SO_2\left( OH\right)_2\]
Explanation: HClO4 (perchloric acid) is strongest.