1.Why are strong acids generally used as standard solutions
in acid-base titrations?
a) The pH at the equivalent point will always be 7
b) They can be used to titrate both strong and weak bases
c) Strong acids form more stable solutions than weak acids
d) The salts of strong acid do not hydrolyse
Explanation: Strong acid can ionise the weak base also.
2. At a certain temperature the dissociation constants
of formic acid and acetic acid are \[1.8\times 10^{-4}\] and
\[1.8\times 10^{-6}\] respectively. The concentration of acetic acid
solution in which the hydrogen ion has the same
concentration as in 0.001 M formic acid solution is equal to
a) 0.001 M
b) 0.01 M
c) 0.1 M
d) 0.0001 M
Explanation:
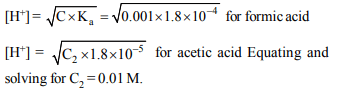
3. A compound having the formula \[NH_{2}CH_{2}COOH\] may behave
a) only as an acid
b) only as a base
c) both as an acid and base
d) Neither acid nor base
Explanation:

4. Water is a
a) protophobic solvent
b) protophilic solvent
c) amphiprotic solvent
d) aprotic solvent
Explanation: H2O can donate and accept protons
5. To \[Ag_{2}CrO_{4}\] solution over its own precipitate, \[CrO_4^{2-}\] ions
are added. This results in
a) increase in \[Ag^{+}\] concentration
b) decrease in \[Ag^{+}\] concentration
c) increase in solubility product
d) shifting of \[Ag^{+}\] ions from the precipitate into the solution
Explanation:

6. To suppress the dissociation of acetic acid, the compound
to be added to it is
a) sodium oxalate
b) sodium acetate
c) sodium carbonate
d) sodium nitrate
Explanation: Common ion effect. CH3COONa will suppress ionisation of CH3COOH.
7. Addition of which chemical will decrease the hydrogen ion
concentration of an acetic acid solution
a) \[NH_{4}Cl\]
b) \[Al_{2}\left(SO_{4}\right)_{3}\]
c) \[AgNO_{3}\]
d) NaCN
Explanation:
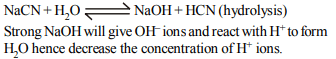
8. Why only \[As^{+3}\] gets precipitated as \[As_{2}S_{3}\] and not \[Zn^{+2}\] as
ZnS when \[H_{2}S\] is passed throgh an acidic solution containing
\[As^{+3}\] and \[Zn^{+2}\] ?
a) Solubility product of \[As_{2}S_{3}\] is less than that of ZnS
b) Enough \[As^{+3}\] are present in acidic medium
c) Zinc salt does not ionise in acidic medium
d) Solubility product changes in presence of an acid
Explanation:
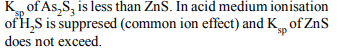
9. The precipitation occurs if ionic concentration is
a) less than solubility product
b) more than solubility product
c) equal to solubility product
d) None of these
Explanation: For precipitation the product of concentration of ions > Ksp
10. The solubility of AgCl will be minimum in
a) 0.001 M \[AgNO_{3}\]
b) pure water
c) \[0.01 M CaCl_{2}\]
d) 0.01 M NaCl
Explanation: Due to common ion effect