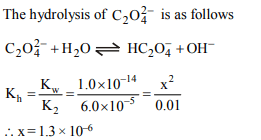1. \[K_{a}\] for HCN is \[5\times10^{-10}\] at 25°C. For maintaining at constant
pH of 9, the volume of 5M KCN solution required to be
added to 10ml of 2M HCN solution is
a) 4 ml
b) 7.95 ml
c) 2 ml
d) 9.3 ml
Explanation:
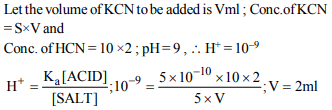
2. An acid-base indicator has \[K_{a}=3.0\times10^{-5}\] . The acid form of
the indicator is red and the basic form is blue. The amount of
change in \[\left[H^{+}\right]\] required to change indicator from 75% red
the 75% blue is
a) \[8\times10^{-5}M\]
b) \[9\times10^{-5}M\]
c) \[1\times10^{-5}M\]
d) \[3\times10^{-4}M\]
Explanation:
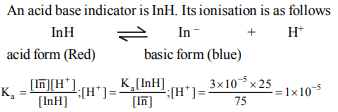
3.Which of the following statements(s) is (are) correct?
a) The pH of \[1.0\times10^{-8}M\] solution of HCl is 8
b) The conjugate base of \[H_{2}PO_4^-\] is \[HPO_4^{2-}\]
c) Autoprotolysis constant of water decreases with
temperature
d) When a solution of a weak monoprotic acid is titrated
against a strong base, at half-neutralisation point \[pH=\left(1/2\right)pK_{a}\]
Explanation:
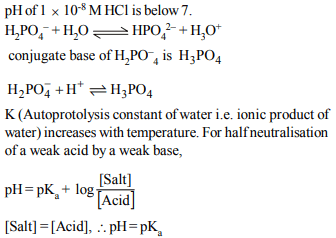
4. The pH of 0.1 M solution of the following salts increases in
the order.
a) \[NaCl < NH_{4}Cl < NaCN < HCl\]
b) \[ HCl < NH_{4}Cl < NaCl < NaCN\]
c) \[ NaCN < NH_{4}Cl < NaCl < HCl\]
d) \[ HCl < NaCN< NaCl < NH_{4}Cl \]
Explanation:
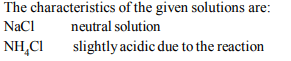
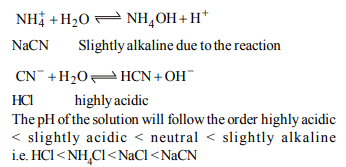
5.What will be the H+ ion concentration in a solution prepared
by mixing 50 mL of 0.20 M NaCl, 25 mL of 0.10 M NaOH and
25 mL of 0.30 N HCl?
a) 0.5 M
b) 0.05 M
c) 0.02 M
d) 0.10 M
Explanation:
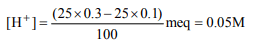
6. Determine the pH of the solution that results from the addition
of 20.00 mL of 0.01 M \[Ca\left(OH\right)_{2} \] to 30.00 mL of 0.01 M HCl.
a) 11.30
b) 10.53
c) 2.70
d) 8.35
Explanation:
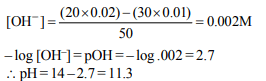
7. Two weak solutions are isohydric when their
a) hydrogen-ion concentrations are the same before mixing
b) hydrogen-ion concentrations are same before and after
mixing
c) degree of dissociation are the same
d) chemical properties are the same
Explanation: Isohydric solutions have the same [H+ ] concentration
8. The dissociation constant of monobasic acids A, B and C
are \[10^{-4}, 10^{-6}\] and \[10^{-10}\] respectively. The concentration of
each is 0.1 M. Which of the following has been arranged in
order of increasing pH?
a) A < B < C
b) C < A < B
c) B < C < A
d) B < A » C
Explanation:
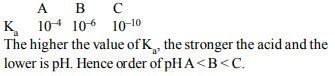
9. Calculate the molar solubility of \[ Fe\left(OH\right)_{2}\] at a pH of 8.00 \[(k_{sp}\] of \[Fe\left(OH\right)_{2}=1.6× 10^{-14})\]
a) 0.06
b) 0.016
c) 0.010
d) 0.16
Explanation:
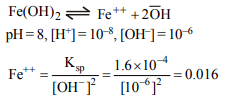
10. \[ K_{1}\] and \[ K_{2}\] for oxalic acid are \[6.5×10^{-2}\] and \[6.1×10^{-5}\] respectively. What will be \[\left[HO^{-}\right]\] in a 0.01 M solution of
sodium oxalate ?
a) \[9.6×10^{-6}\]
b) \[1.4×10^{-1}\]
c) \[1.3×10^{-6}\]
d) \[1.3×10^{-8}\]
Explanation: