1. The solubility product of \[Ag_{2}CrO_{4}\] is \[32 × 10^{-12} \] . What is the concentration of
\[CrO_4^{-2}\] ions in that solution (in g ions \[L^{-1})\]
a) \[2 × 10^{-4}\]
b) \[8 × 10^{-4}\]
c) \[8 × 10^{-8}\]
d) \[16 × 10^{-4}\]
Explanation:
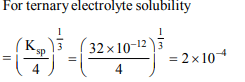
2. The solubility product of silver chloride is \[1.8 × 10^{-10}\] at 298
K. The solubility of AgCl in 0.01 M HCl solution in mol/ \[dm^{3}\] is
a)\[2.4 × 10^{-9}\]
b) \[3.6 × 10^{-8}\]
c) \[0.9 × 10^{-10}\]
d \[1.8 × 10^{-8}\]
Explanation:
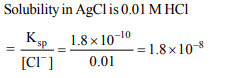
3. \[K_{sp}\] for \[HgSO_{4}\] is \[6.4× 10^{-5}\] , then solubility of the salt is
a)\[8 × 10^{-6}\]
b) \[8 × 10^{-3}\]
c) \[4.6 × 10^{-5}\]
d) None of these
Explanation:
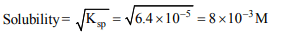
4. The maximum amount of \[BaSO_{4}\] precipitated on mixing \[BaCl_{2}\] (0.5 M) with \[H_{2}SO_{4}\] (1M) will correspond to
a) 0.5 M
b) 1.0 M
c) 1.5 M
d) 2.0 M
Explanation:
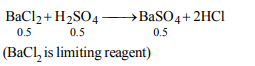
5. At 20°C, the Ag+ ion concentration in a saturated solution
of \[Ag_{2}CrO_{4}\] is \[1.5 × 10^{-4}\] mole/litre. At 20°C, the solubility
product of \[Ag_{2}CrO_{4}\] will be
a) \[3.3750 × 10^{-12}\]
b) \[1.6875 × 10^{-10}\]
c) \[1.6875 × 10^{-12}\]
d) \[1.6875 × 10^{-11}\]
Explanation:
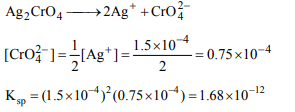
6. The solubility of BaSO4 in water is \[2.33 × 10^{-3} g L^{-1}\] . Its solubility product will be (molecular weight of
\[BaSO_{4}\] = 233)
a) \[1 × 10^{-5}\]
b) \[1 × 10^{-10}\]
c) \[1 × 10^{-15}\]
d) \[1 × 10^{-20}\]
Explanation:
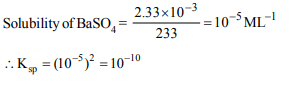
7. What is the minimum concentration of \[SO_4^{2-}\] required to
precipitate \[BaSO_{4}\] in a solution containing \[1.0 × 10^{-4}\] mole of \[Ba^{2+}\] ? \[K_{sp}\] for \[BaSO_{4}=4 × 10^{-10}\]
a) \[4 × 10^{-10}M\]
b) \[2 × 10^{-7}M\]
c) \[4 × 10^{-6}M\]
d) \[2 × 10^{-3}M\]
Explanation:
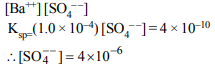
8. Solubility of an \[MX_{2}\] type electrolyte is \[0.5 × 10^{-4}\] mole/lit,
then \[K_{sp}\] of the electrolyte is
a) \[5 × 10^{-12}\]
b) \[25 × 10^{-10}\]
c) \[1 × 10^{-13}\]
d) \[5 × 10^{-13}\]
Explanation:
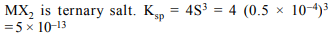
9.The pH of blood does not appreciably change by a small
addition of acid or a base because blood
a) contains serum protein which acts as buffer
b) contains iron as a part of the molecule
c) can be easily coagulated
d) is body fluid
Explanation: Blood contains serum protein which acts as buffer
10.The pH of solutions A, B, C, D are respectively 9.5, 2.5, 3.5,
5.5. The most acidic solution is
a) A
b) B
c) C
d) D
Explanation: Smaller the pH, the more the acidic character