1. When rain is accompanied by a thunderstorm, the collected
rain water will have a pH value
a) slightly lower than that of rain water without
thunderstorm
b) slightly higher than that when the thunderstorm is not
there
c) uninfluenced by occurrence of thunderstorm
d) depends on the amount of dust in air
Explanation:
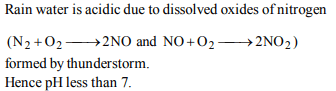
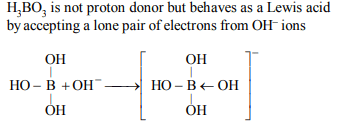
2. \[H_{3}BO_{3}\] is
a) monobasic and weak Lewis acid
b) monobasic and weak Bronsted acid
c) monobasic and strong Lewis acid
d) tribasic and weak Bronsted acid
Explanation:
3. A solution which is \[10^{-3}M\] each in \[Mn^{2+},Fe^{2+},Zn^{2+}\] and \[Hg^{2+}\] is treated with \[10^{-6}M\] sulphide ion. If \[K_{sp}\] of
MnS,FeS, ZnS and HgS are \[10^{-15},10^{-23},10^{-20}\] and \[10^{-54}\] respectively which one will precipitate first
a) FeS
b) MgS
c) HgS
d) ZnS
Explanation: Since Ksp of HgS is minimum among others, HgS will precipitate first.
4. A weak acid, HA, has a \[K_{a}\] of \[1.0\times10^{-5}\] . If 0.1 mole of this
acid dissolved in one litre of water, the percentage of acid
dissociated at equilbrium is closest to
a) 1.0%
b) 99.9%
c) 0.1%
d) 99.0%
Explanation:
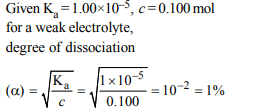
5. Calculate the pOH of a solution at 25°C that contains
\[1\times10^{-10}M\] of hydronium ions, i.e. \[H_{3}O^{+}\] .
a) 4.0
b) 9.0
c) 1.0
d) 7.0
Explanation:
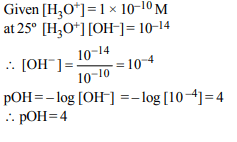
6. Equal volumes of three acid solutions of pH 3, 4 and 5 are
mixed in a vessel. What will be the H+ ion concentration in
the mixture ?
a) \[1.11\times10^{-4}M\]
b) \[3.7\times10^{-4}M\]
c) \[3.7\times10^{-3}M\]
d) \[1.11\times10^{-3}M\]
Explanation:
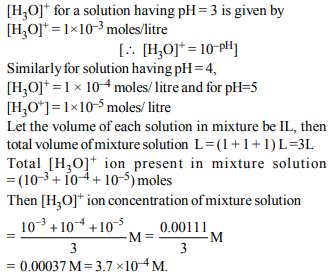
7. If the concentration of OH– ions in the reaction
\[Fe \left(OH\right)_{3}\left(s\right)\rightleftharpoons Fe^{3+}(aq)+3OH^{-}\left(aq\right)\]
is
decreased by \[\frac{1}{4}\] times, then equilibrium concentration of \[Fe^{3+}\]
will increase by :
a) 8 times
b) 16 times
c) 64 times
d) 4times
Explanation:

8. Equimolar solutions of the following were prepared in water
separately. Which one of the solutions will record the highest
pH ?
a) \[SrCl_{2}\]
b) \[BaCl_{2}\]
c) \[MgCl_{2}\]
d) \[CaCl_{2}\]
Explanation: The highest pH will be recorded by the most basic solution. The basic nature of hydroxides of alkaline earth metals increase as we move from Mg to Ba and thus the solution of BaCl2 in water will be most basic and so it will have highest pH
9.The dissociation constants for acetic acid and HCN at 25°C
are \[1.5\times10^{-5}\] and \[4.5 × 10^{-10}\] respectively. The equilibrium
constant for the equilibrium \[CN^{-}+CH_{3}COOH\rightleftharpoons HCN + CH_{3}COO^{-}\]
would be:
a) \[3.0 × 10^{-5}\]
b) \[3.0 × 10^{-4}\]
c) \[3.0 × 10^{4}\]
d) \[3.0 × 10^{5}\]
Explanation:
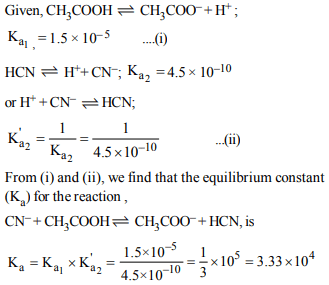
10. Which of the following molecules acts as a Lewis acid ?
a) \[\left(CH_{3}\right)_{2}O\]
b) \[\left(CH_{3}\right)_{3}P\]
c) \[\left(CH_{3}\right)_{3}N\]
d) \[\left(CH_{3}\right)_{3}B\]
Explanation: (CH3 )3 B - is an electron deficient, thus behave as a lewis acid