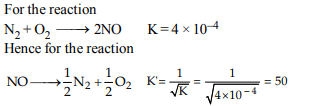1. The pKa of a weak acid, HA, is 4.80. The pKb of a weak base,
BOH, is 4.78. The pH of an aqueous solution of the
correspondng salt, BA, will be
a) 9.58
b) 4.79
c) 7.01
d) 9.22
Explanation:
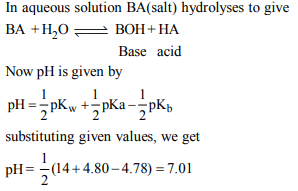
2. Solid \[Ba\left(NO_3\right)_2\] is gradually dissolved in a 1.0 × 10– 4 M \[Na_2CO_3\] solution. At what concentration of \[Ba^{2+}\] will a
precipitate begin to form? (\[K_{sp}\] for \[BaCO_3= 5.1 × 10^{-9})\]
a) \[5.1 × 10^{-5}M\]
b) \[8.1 × 10^{-8}M\]
c) \[8.1 × 10^{-7}M\]
d) \[4.1 × 10^{-5}M\]
Explanation:
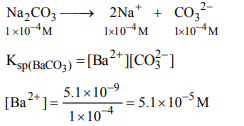
3.Three reactions involving \[H_{2}PO_4^-\] are given below
(i) \[H_{3}PO_4+H_{2}O\rightarrow H_{3}O^+ +H_{2}PO_4^-\]
(ii) \[H_{2}PO_4^-+H_{2}O\rightarrow HPO_4^{2-} +H_{3}O^{+}\]
(iii) \[H_{2}PO_4^-+OH^{-}\rightarrow H_{3}PO_{4} +O^{2-}\]
In which of the above does \[H_{2}PO_4^-\] act as an acid ?
a) (ii) only
b) (i) and (ii)
c) (iii) only
d) (i) only
Explanation:
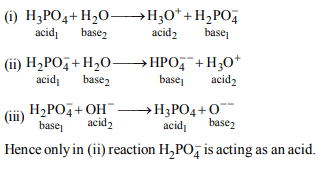
4. In aqueous solution the ionization constants for carbonic acid are \[K_{1}= 4.2 × 10^{-7}\] and \[K_{2}= 4.8 × 10^{-11}\] .
Select the correct statement for a saturated 0.034 M solution
of the carbonic acid.
a) The concentration of \[CO_3^{2-}\] is 0.034 M.
b) The concentration of \[CO_3^{2-}\] is greater than that of \[HCO_3^-\]
c) The concentrations of \[H^{+}\] and \[HCO_3^-\] are approximately equal
d) The concentration of \[H^{+}\] is double that of \[CO_3^{2-}\] .
Explanation:
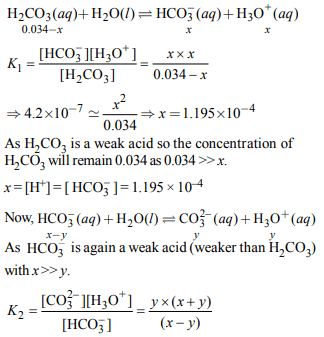
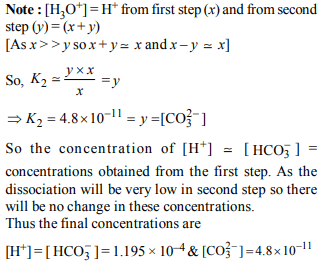
5. Solubility product of silver bromide is \[5.0 × 10^{-13}\] . The
quantity of potassium bromide (molar mass taken as 120 g \[mol^{-1})\] to be added to 1 litre of 0.05 M solution of silver nitrate
to start the precipitation of AgBr is
a) \[1.2 × 10^{-10}g\]
b) \[1.2 × 10^{-9}g\]
c) \[6.2 × 10^{-5}g\]
d) \[5.0 × 10^{-8}g\]
Explanation:
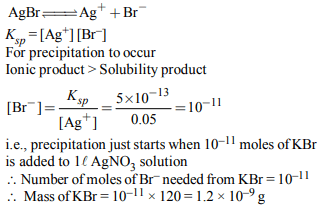
6. At 25°C, the solubility product of \[Mg\left(OH\right)_{2}\] is \[1.0 × 10^{-11}\] . At
which pH, will \[Mg^{2+}\] ions start precipitating in the form of \[Mg\left(OH\right)_{2}\] from a solution of 0.001 M \[Mg^{2+}\] ions?
a) 9
b) 10
c) 11
d) 8
Explanation:
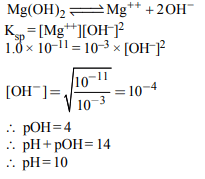
7. An acid HA ionises as \[HA\rightleftharpoons H^{+}+ A ^{-}\]
The pH of 1.0 M solution is 5. Its dissociation constant would
be :
a) 5
b) \[5 \times10 ^{-8}\]
c) \[1 \times10 ^{-5}\]
d) \[1 \times10 ^{-10}\]
Explanation:
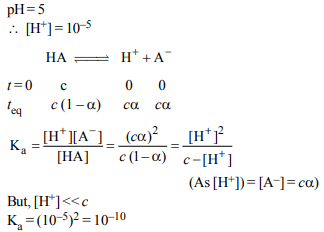
8. The \[K_{sp}\] for \[Cr\left(OH\right)_{3}\] is \[1.6 × 10^{-30}\] . The solubility of this
compound in water is :
a) \[\sqrt[4]{1.6 × 10^{-30}}\]
b) \[\sqrt[4]{1.6 × 10^{-30}/27}\]
c) \[1.6 × 10^{-30/27}\]
d) \[\sqrt{1.6 × 10^{-30}}\]
Explanation:
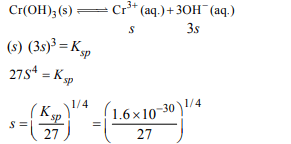
9. A vessel at 1000 K contains \[CO_{2}\] with a pressure of 0.5 atm.
Some of the \[CO_{2}\] is converted into CO on the addition of
graphite. If the total pressure at equilibrium is 0.8 atm, the
value of K is :
a) 1.8 atm
b) 3 atm
c) 0.3 atm
d) 0.18 atm
Explanation:
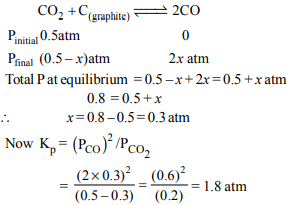
10. The equilibrium constant \[\left(K_{c}\right)\] for the reaction
\[N_{2}\left(g\right)+O_{2}\left(g\right)\rightarrow 2NO\left(g\right)\]
at temperature T is \[4 × 10^{-4}\] . The
value of \[K_{c}\] for the reaction \[NO\left(g\right)\rightarrow\frac{1}{2}N_{2}\left(g\right)+\frac{1}{2} O_{2}\left(g\right)\]
at the same temperature is:
a) 0.02
b) \[2.5 × 10^{2}\]
c) \[4 × 10^{-4}\]
d) 50.0
Explanation: