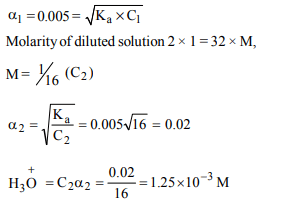1. Calculate the pH of a solution containing 0.1 M \[HCO_3^-\] and
0.2 M \[CO_3^{2-}\]
\[[K_{1}\left(H_{2}CO_{3}\right)= 4.2×10^{-7}\times 10\]
and \[K_{2}\left(HCO_3^-\right)= 4.8×10^{-11}]\]
a) 3.18
b) 10.62
c) 6.62
d) 9.31
Explanation:
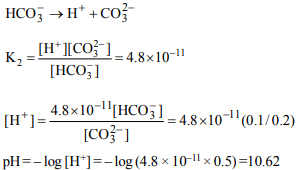
2. At what pH will a \[1×10^{-4}M\] solution of an indicator will (\[K_{b}\]
indicator) = \[1×10^{-11}\] change colour?
a) 7.0
b) 3.0
c) 5.5
d) 11.0
Explanation:
3. Calculate the pH of a 0.01 M \[NaHCO_{3}\] solution \[\left[K_{1}\left(H_{2}CO_{3}\right)=4×10^{-7},K_{2}\left(HCO_3^-\right)= 4.8×10^{-11}\right]\]
a) 9.38
b) 6.38
c) 8.38
d) 7.38
Explanation: 8.38
4. Blood plasma is maintained at a pH of 7.4 largely by the
a) \[HCO_{3}^{-} /H_{2}CO_{3}\] buffer
b) \[HPO_{4}^{2-} /PO_4^{3-}\] buffer
c) \[HCO_{3}^{-} /H_{2}CO_{3}\]
and \[HPO_4^{2-}/H_{2}PO_4^-\] buffer
d) \[HPO_{4}^{2-} /H_{3}PO_4\] and haemoglobin buffers
Explanation:
5. In the titration of a weak diprotic acid \[\left(H_{2} A\right)\] with a strong
base (NaOH), \[\left[H^{+}\right]\] is given by
a) \[\sqrt{K_{_{a_{1}}}C_{a}}\]
b) \[K_{a}\sqrt{C_{_{a_{1}}}}\]
c) \[K_{a}C_{_{a_{1}}}\]
d) \[K_{_{a_{1}}}\]
Explanation:
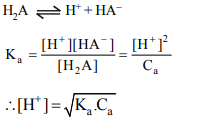
6. The pH of a solution prepared by mixing 2.0 ml of a strong
acid (HCl) solution of pH 3.0 and 3.0 ml of a strong base
(NaOH) of pH 10.0
a) 4.5
b) 3.4
c) 2.5
d) 6.5
Explanation:
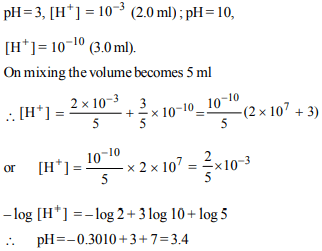
7. For a sparingly soluble salt ApBq, the relationship of its
solubility product \[Ls\rightarrow K_{sp}\] with its solubility (S) is
a) \[Ls\rightarrow K_{sp}=S^{pq}\left(pq\right)^{p+q}\]
b) \[Ls= S^{p+q}.P^{P}q^{q}\]
c) \[Ls\rightarrow K_{sp}=S^{p+q}p^{q}q^{p}\]
d) \[Ls\rightarrow K_{sp}=S^{pq}p^{P}q^{q}\]
Explanation:
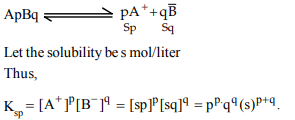
8. The correct order of increasing solubility of AgCl in (A) water,
(B) 0.1 M NaCl, (C) 0.1 M, \[BaCl_{2}\] , (D) 0.1 M \[NH_{3}\] is
a) D > A > B > C
b) D > C > B > A
c) B > A > D > C
d) A > D > B > C
Explanation:
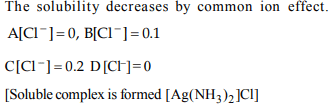
9. If \[pK_{b}\] for fluoride ion at 25°C is 10.83, the ionisation constant
of hydrofluoric acid in water at this temperature is
a) \[3.52 × 10^{-3}\]
b) \[6.75 × 10^{-4}\]
c) \[5.38 × 10^{-2}\]
d) \[1.74 × 10^{-5}\]
Explanation:
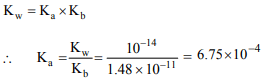
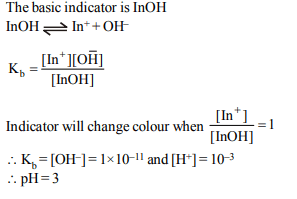
10.The degree of dissociation of 0.1M weak acid HA is 0.5%. If
2 ml of 1.0 M HA solution is diluted to 32 ml the degree of
dissociation of acid and \[H_{3}O^{+}\] ion concentration in the
resulting solution will be respectively
a) 0.02 and \[3.125 × 10^{-4}\]
b) \[1.25 × 10^{-3}\] and 0.02
c) 6.02 and \[1.25 × 10^{-3}\]
d) 0.02 and \[8.0 × 10^{-12}\]
Explanation: