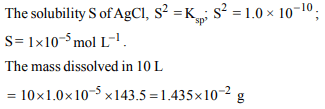1. The dissociation constants of a weak acid HA and weak
base BOH are \[2 × 10^{-5}\] and \[5 × 10^{-6}\] respectively. The
equilibrium constant for the neutralisation reaction of the
two is
a) \[1.0 × 10^{4}\]
b) \[1.0 × 10^{-4}\]
c) \[1.0 × 10^{-10}\]
d) \[2.5 × 10^{-1}\]
Explanation:
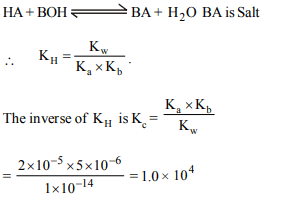
2. The ionisation constant of an acid-base indicator
(a weak acid) is \[1.0 × 10^{-6}\] . The ionised form of the indicator
is red whereas the unionised form is blue the pH change
required to alter the colour of the indicator from 80% blue to
80% red is
a) 1.40
b) 1.20
c) 0.80
d) 2.00
Explanation:
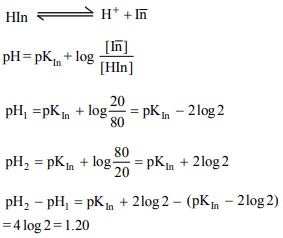
3. The pH at the equivalence point in the titration of 25 ml of
0.10 M formic acid with a 0.1 M NaOH solution (given that
\[pK_{a}\] of formic acid = 3.74)
a) 8.74
b) 8.37
c) 4.74
d) 6.06
Explanation:
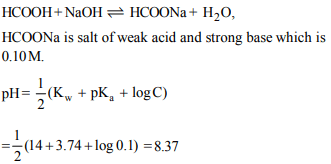
4.The pH of a weak monoacid base at 80% neutralisation with
a strong acid in a dilute solution is 7.40. The ionisation
constant of the base is
a) \[1.6 × 10^{-7}\]
b) \[1.0 × 10^{-5}\]
c) \[1.0 × 10^{-6}\]
d) \[2.0× 10^{-7}\]
Explanation:
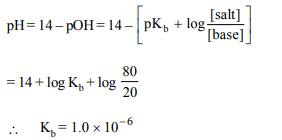
5. What will be the volume of 1 M NH3 and 1 M HCl required
to prepare 300 ml of a buffer of pH = 9.26 \[(pK_{a}\] = 9.26 for \[NH_4^+)\]
a) 225 ml, 75 ml
b) 200 ml, 100 ml
c) 100 ml, 200 ml
d) 150 ml, 150 ml
Explanation:
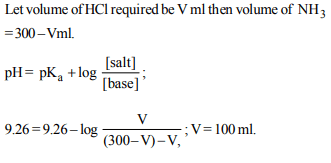
6. \[K_{sp}\] of \[M(OH)_{2}\] is \[3.2 × 10^{-11}\] . The pH of saturated solution
in water is
a) 3.40
b) 10.30
c) 10.60
d) 3.70
Explanation:
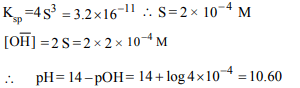
7. The concentration of hydroxyl ion in a solution left after
mixing 100 ml of 0.1 M \[MgCl_{2}\] and 100 ml of 0.2 M NaOH \[(K_{sp}\] of \[Mg \left(OH\right)_{2} = 1.2\times 10^{-11}) \] is
a) \[2.8\times10^{-4}\]
b) \[2.8\times10^{-3}\]
c) \[2.8\times10^{-2}\]
d) \[2.8\times10^{-5}\]
Explanation:
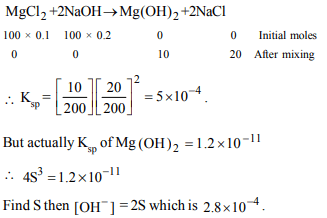
8. In the titration of monoacid base (weak) with a strong acid
the pH at half of the equivalence point
a) \[14 – pK_{b}\]
b) \[= pK_{b}\]
c) \[7 – pK_{b}\]
d) \[7+ pK_{b}\]
Explanation:

9. The \[pK_{a}\] value for the \[A\rightarrow B, B\rightarrow C\] and \[C\rightarrow D\] dissociations are 2.09, 3.86 and 9.82 respectively. Since only
B has an equal number of positive and negative charges, the
value of the isoelectric point is
a) 5.26
b) 2.98
c) 3.86
d) 15.77
Explanation:
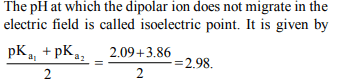
10. Mass loss of 1.0000 g of the AgCl \[\left(K_{sp}=1.0× 10^{-10}\right)\] on
repeated washing with 10 L of water is (Ag = 108,Cl = 35.5)
a) \[1.43 × 10^{-2}g\]
b) \[1.43 × 10^{-3}g\]
c) \[1.0 × 10^{-4}g\]
d) \[1.34 × 10^{-3}g\]
Explanation: