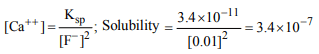1. The pH of a 0.1 molar solution of the acid HQ is 3. The value
of the ionization constant, \[K_{a}\] of the acid is :
a) \[3 × 10^{-1}\]
b) \[1 × 10^{-3}\]
c) \[1 × 10^{-5}\]
d) \[1 × 10^{-7}\]
Explanation:
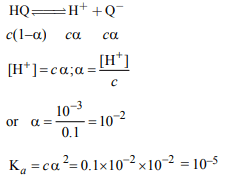
2. How many litres of water must be added to 1 litre an aqueous
solution of HCl with a pH of 1 to create an aqueous
solution with pH of 2 ?
a) 0.1 L
b) 0.9 L
c) 2.0 L
d) 9.0 L
Explanation:
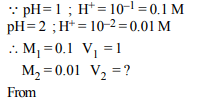
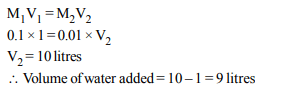
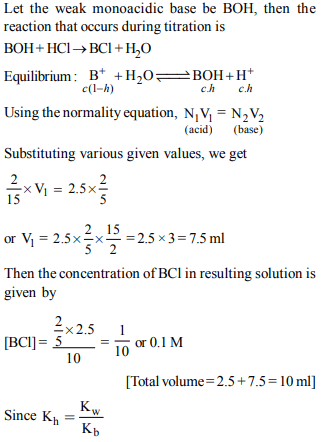
3. 2.5 ml of (2/5) M weak monoacidic base (\[K_{b}= 1 × 10^{-12}\] at 25°)
is titrated with (2/15) M HCl in water at 25°C. The
concentration of H+ at equivalence point is (\[K_{w}= 1 × 10^{-14}\] at
25°C)
a) \[3.7 × 10 ^{-14}M\]
b) \[3.2 × 10 ^{-7}M\]
c) \[3.2 × 10 ^{-2}M\]
d) \[2.7 × 10 ^{-2}M\]
Explanation:
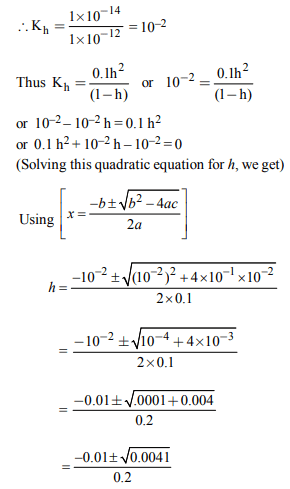
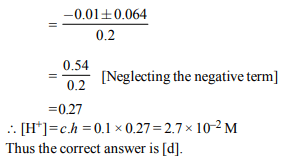
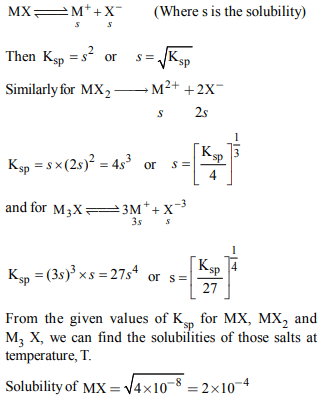
4. Solubility product constant \[\left(K_{sp}\right)\] of salts of types MX, \[MX_{2}\]
and \[M_{3}X\] at temperature T are \[4.0 × 10 ^{-8}\] , \[3.2 × 10 ^{-14}\] and \[2.7 × 10 ^{-15}\] , respectively. Solubilities (mol \[dm^{-3})\] of the salts
at temperature 'T' are in the order –
a) \[MX > MX_{2} > M_{3}X\]
b) \[ M_{3}X > MX_{2}>MX \]
c) \[ MX_{2} > M_{3}X>MX \]
d) \[MX > M_{3}X > MX_{2}\]
Explanation:
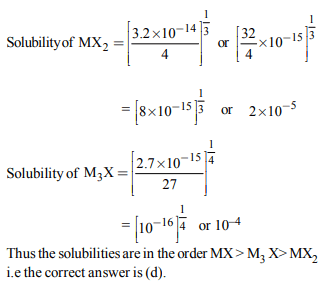
5.How many gms of \[CaC_{2} O_{4}\] will dissolve in litre of saturated
solution. \[K_{sp}\] of \[CaC_{2} O_{4}\] is \[2.5 \times 10^{-9}mol^{2}lit ^{-2}\]
a) 0.0064 g
b) 0.0128 g
c) 0.0032 g
d) None of these
Explanation:
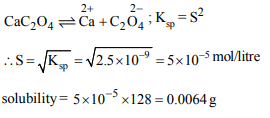
6. For \[NH_{3},K_{b}=1.8 × 10^{-5}\] and \[K_{a}\] for \[NH_4^+\] would be
a) \[1.8 × 10^{-5}\]
b) \[5.56 × 10^{5}\]
c) \[1.8 × 10^{10}\]
d) \[5.56 × 10^{-10}\]
Explanation:
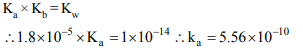
7. The pH of a solution which is 0.1 M in HA and 0.5 M in NaA.
\[K_{a}\] for HA is \[1.8 × 10^{-6}\]
a) 5.44
b) 6.44
c) 6.0
d) 4.73
Explanation:
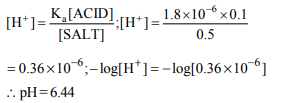
8. The pH of a solution obtained by mixing of 100.0 ml of 0.1 M
HCl and 100 ml of 0.2 M \[NH_{3}\] , \[K_{b}\] for \[NH_{3}\] is \[1.8 × 10^{-5}\]
a) 4.74
b) 9.26
c) Less than 7
d) None of these
Explanation:
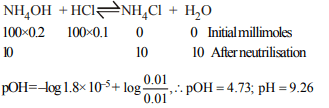
9. The percentage hydrolysis of 0.15 M solution of ammonium acetate, \[K_{a}\] for \[CH_{3}COOH\] is \[1.8 × 10^{-5}\] and \[K_{b}\] for \[NH_{3}\] is
\[1.8 × 10^{-5}\]
a) 0.556
b) 4.72
c) 9.38
d) 5.56
Explanation:
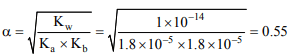
10. The solubility of \[CaF_{2}\] in 0.01 M solution of NaF. The \[K_{sp}\] of
\[CaF_{2}\] is \[3.4× 10^{-11}\]
a) \[3.4\times10^{-7}\] mol / l
b) \[3.4\times10^{-5}\] mol / l
c) 3.4Mol / l
d) None of these
Explanation: