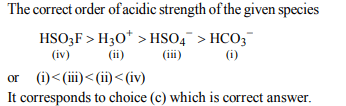1. The solubility product of a salt having general formula \[MX_{2}\] ,
in water is : \[4 \times 10^{-12} \] . The concentration of \[M^{2+} \] ions in the
aqueous solution of the salt is
a) \[4.0 \times 10^{-10} M\]
b) \[1.6 \times 10^{-4} M\]
c) \[1.0 \times 10^{-4} M\]
d) \[2.0 \times 10^{-6} M\]
Explanation:
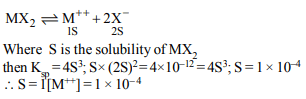
2. Hydrogen ion concentration in mol/L in a solution of
pH = 5.4 will be :
a) \[3.98 \times 10^{-6}\]
b) \[3.68 \times 10^{-6}\]
c) \[3.88 \times 10^{6}\]
d) \[3.98 \times 10^{8}\]
Explanation:
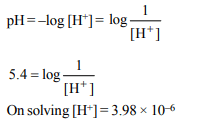
3. What is the conjugate base of \[OH^{-}\] ?
a) \[O^{2-}\]
b) \[O^{-}\]
c) \[H_{2}O\]
d) \[O_{2}\]
Explanation:
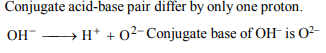
4. Which of the following statements is true?
a) \[ HClO_{4}\] is a weaker acid than \[ HClO_{3}\]
b) \[ HNO_{3}\] is a stronger acid than \[ HNO_{2}\]
c) \[ H_{3}PO_{3}\] is a stronger acid than \[ H_{2}SO_{3}\]
d) In aqueous medium HF is a stronger acid than HCl
Explanation:

5. Given the data at 25ºC
\[Ag + I^{-}\rightarrow AgI + e^{-}\] \[E^{\circ}= 0.152 V\]
\[Ag \rightarrow Ag^+ + e^{-}\] \[E^{\circ}= -0.800 V\]
What is the value of log Ksp for AgI? (2.303 RT/ F = 0.059 V)
a) –37.83
b) –16.13
c) –8.12
d) +8.612
Explanation:
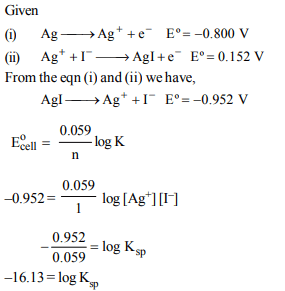
6. The first and second dissociation constants of an acid H2A
are \[1.0 × 10^{-5}\] and \[5.0 × 10^{-10}\] respectively. The overall
dissociation constant of the acid will be
a) \[0.2 × 10^{5}\]
b) \[5.0 × 10^{-5}\]
c) \[5.0 × 10^{15}\]
d) \[5.0 × 10^{-15}\]
Explanation:
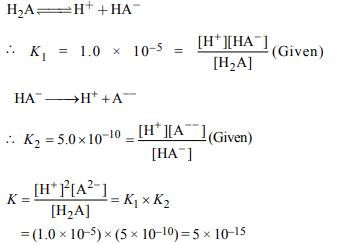
7. The \[pK_{a}\] of a weak acid (HA) is 4.5. The pOH of an aqueous
buffer solution of HA in which 50% of the acid is ionized is
a) 7.0
b) 4.5
c) 2.5
d) 9.5
Explanation:
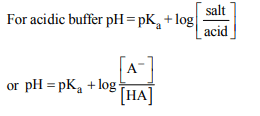
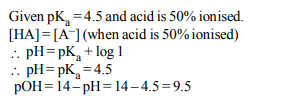
8. In a saturated solution of the sparingly soluble strong
electrolyte \[AgIO_{3}\] (molecular mass = 283) the equilibrium
which sets is \[AgIO_{3}(s)\rightleftharpoons Ag^{+}\left(aq\right)+IO_3^-\left(aq\right)\]
If the
solubility product constant \[K_{sp}\] of \[AgIO_{3}\] at a given
temperature is \[1.0 × 10^{-8}\] , what is the mass of \[AgIO_{3}\] contained
in 100 ml of its saturated solution?
a) \[1.0 × 10^{-4}g\]
b) \[28.3 × 10^{-2}g\]
c) \[2.83 × 10^{-3}g\]
d) \[1.0 × 10^{-7}g\]
Explanation:
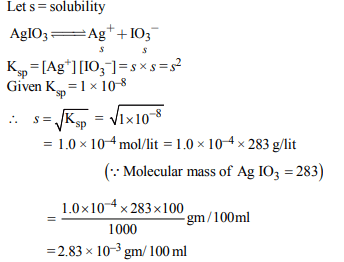
9. For the following three reactions a, b and c, equilibrium
constants are given:
(i) \[CO(g) +H_{2}O(g)\rightleftharpoons CO_{2}(g)+ H_{2}(g);K_{1}\]
(ii) \[CH_{4}(g) +H_{2}O(g)\rightleftharpoons CO(g) +3H_{2}(g);K_{2}\]
(iii) \[CH_{4}(g) +2H_{2}O(g)\rightleftharpoons CO_{2}(g) +4H_{2}(g);K_{3}\]
a) \[K_{1}\sqrt{K_{2}}=K_{3}\]
b) \[K_{2}K_{3}=K_{1}\]
c) \[K_{3}=K_{1}K_{2}\]
d) \[K_{3}.K_2^3=K_1^2\]
Explanation: Reaction (c) can be obtained by adding reactions (a) and (b) therefore K3 = K1 . K2
Hence (c) is the correct answer
10. Four species are listed below:
i. \[HCO_3^-\]
ii. \[H_3O^{+}\]
iii. \[HSO_4^-\]
iv. \[HSO_3F\]
Which one of the following is the correct sequence of their
acid strength?
a) iv < ii < iii < i
b) ii < iii < i < iv
c) i < iii < ii < iv
d) iii < i < iv < ii
Explanation: