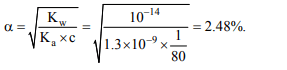1. The pH of 0.0001 M NaOH is
a) 4
b) 10
c) 12
d) 11
Explanation:
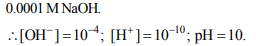
2.The pOH value of a solution whose hydroxide ion
concentration is \[6.2 × 10^{-9}\] mol/litre is
a) 8.21
b) 6.21
c) 7.75
d) 7.21
Explanation:
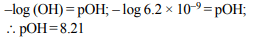
3. The pH of a solution obtained by mixing 50 ml of 0.4 N HCl
and 50 ml of 0.2 N NaOH is
a) – log 2
b) – log 0.2
c) 1.0
d) 2.0
Explanation:
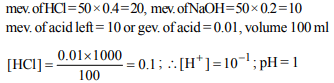
4. The pH of 1.0 M aqueous solution of a weak acid HA is 6.0.
Its dissociation constant is
a) \[1.0 × 10^{-12} \]
b) \[1.0 × 10^{-6} \]
c) 1.0
d) 6.0
Explanation:
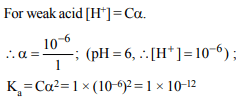
5.The pH of a solution is increased from 3 to 6; its H+ ion
concentration will be
a) reduced to half
b) doubled
c) reduced by 1000 times
d) increased by 1000 times
Explanation:
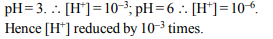
6. When \[CO_{2}\] dissolves in water, the following equilibrium is
established \[CO_{2}+2H_{2}O\rightleftharpoons H_{3}O^{+}+HCO_3^-\]
for which the equilibrium constant is \[3.8 × 10^{-7}\] and pH = 6.0.
The ratio of \[\left[HCO_3^-\right]\] to \[\left[CO_{2}\right]\] would be
a) \[3.8 × 10^{-13} \]
b) \[3.8 × 10^{-1} \]
c) 6.0
d) 13.4
Explanation:
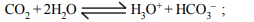
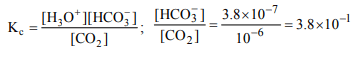
7. An example of a salt that will not hydrolyse is
a) \[NH_{4}Cl\]
b) KCl
c) \[CH_{3}COONH_{4}\]
d) \[CH_{3}COOK\]
Explanation: Salt of strong acid and strong base give neutral solution (pH = 7). Hence such salts are not hydrolysed. pH = 7
8. pH of 2 M NaCl will be
a) 3
b) 6.5
c) 7
d) 10
Explanation: NaCl solution (salt of strong base and strong acid) hence pH = 7
9. Dissociation constant of NH4OH is \[1.8\times 10^{-5}\] . The
hydrolysis constant of \[NH_{4}Cl\] would be
a) \[1.80\times 10^{-19}\]
b) \[5.55\times 10^{-10}\]
c) \[5.55\times 10^{-5}\]
d) \[1.80\times 10^{-5}\]
Explanation:
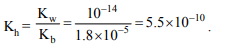
10. What is the percentage hydrolysis of NaCN in N/80 solution
when the dissociation constant for HCN is \[1.3\times 10^{-9}\] and
\[K_{W}=1.0\times 10^{-14}\]
a) 2.48
b) 5.26
c) 8.2
d) 9.6
Explanation: