1. A monoprotic acid in a 0.1 M solution ionizes to 0.001%. Its
ionisation constant is
a) \[ 1.0 × 10^{-3}\]
b) \[ 1.0 × 10^{-6}\]
c) \[ 1.0 × 10^{-8}\]
d) \[ 1.0 × 10^{-11}\]
Explanation:
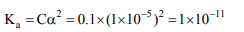
2. The concentration of water molecules in one litre of water at
298 K is
a) \[ 10^{-7}M\]
b) 55.5 M
c) 5.55 M
d) 7.26 M
Explanation:
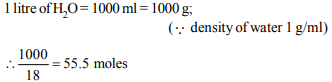
3.Which of the following will occur if 0.1 M solution of a weak
acid is diluted to 0.01 M at constant temperature
a) \[ \left[ H^{+}\right]\] will decrease to 0.01 M
b) pH will decrese
c) Percentage ionization will increase
d) \[K_{a}\] will increase
Explanation: Ostwald's dilution law, dilution increases ionisation
4. Which of the following is not a Lewis base?
a) \[CH_{4}\]
b) \[C_{2}H_{5}OH\]
c) Acetone
d) Sec amine
Explanation: Methane cannot donate electrons. While others can donate electrons. Lewis bases are electron doners
5. Which of the following is not a Lewis acid?
a) \[BF_{3}\]
b) \[AlCl_{3}\]
c) \[FeCl_{3}\]
d) \[PH_{3}\]
Explanation: PH3 is Lewis base and not Lewis acid.
6.In the given anions, the strongest Bronsted base is
a) \[ClO^{-}\]
b) \[ClO_2^-\]
c) \[ClO_3^-\]
d) \[ClO_4^-\]
Explanation: The weaker the conjugate acid, the stronger is the base and vice versa. HClO is a weak acid, hence ClO- is a strong base.
7. Which of the following can act as both Bronsted acid and
Bronsted base?
a) \[Na_{2}CO_{3}\]
b) \[OH^-\]
c) \[HCO_3^-\]
d) \[NH_{3}\]
Explanation:
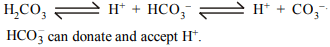
8.Which of the following is the strongest Lewis acid?
a) \[BI_{3}\]
b) \[BBr_{3}\]
c) \[BCl_{3}\]
d) \[BF_{3}\]
Explanation: Back bond formation decreases with increase in size of halogen atom. Hence B in BI3 is more electron deficient
9. The strongest conjugate base is
a) \[NO_3^-\]
b) \[Cl^-\]
c) \[SO_4^2-\]
d) \[CH_{3}COO^{-}\]
Explanation: CH3COOH is a weak acid, hence CH3COO- is a strong base
10. A base, as defined by Bronsted theory, is a substance which
can
a) lose a pair of electrons
b) donate protons
c) gain a pair of electrons
d) accept protons
Explanation: Base accepts protons and acid donates protons.