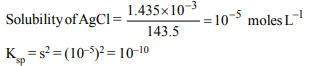1. The solubility of \[PbCl_{2}\] is
a) \[\sqrt{K_{sp}}\]
b) \[\left(K_{sp}\right)^{1/3}\]
c) \[\left(K_{sp}/4\right)^{1/3}\]
d) \[\left(8K_{sp}\right)^{1/2}\]
Explanation:
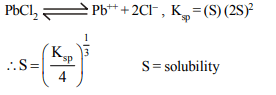
2. The solubility of calcium phosphate in water is
x mol \[L^{-1}\] at 25°C. Its solubility product is equal to
a) \[108 x^{2}\]
b) \[36 x^{3}\]
c) \[36 x^{5}\]
d) \[108 x^{5}\]
Explanation:
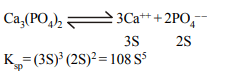
3.Which of the following sulphides has the lowest solubility
product?
a) FeS
b) MnS
c) PbS
d) ZnS
Explanation: Sulphides of IIA and IIB have low value of Ksp .
4. The \[K_{sp}\] of CuS, \[Ag_{2}S\] and HgS are \[10^{-31},10^{-44} \] and \[10^{-54}\] respectively. The solubility of these
sulphides are in the order
a) \[Ag_{2}S > CuS > HgS\]
b) AgS > HgS > CuS
c) \[ HgS> Ag_{2}S >CuS\]
d) \[ CuS > Ag_{2}S >HgS\]
Explanation: For CuS solubility is (10–31)1/2;
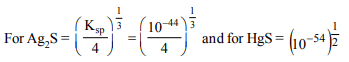
5.Which of the following on reaction with \[H_{2}S\] does not
produce metallic sulphide?
a) \[CdCl_{2}\]
b) \[ZnCl_{2}\]
c) \[COCl_{2}\]
d) \[CuCl_{2}\]
Explanation: COCl2 is phosgene hence will not give any metal sulphide.
6. What is the correct representation for the solubility product
of \[SnS_{2}\] ?
a) \[\left[Sn^{2+}\right]\left[S^{2-}\right]^{2}\]
b) \[\left[Sn^{4+}\right]\left[S^{2-}\right]^{2}\]
c) \[\left[Sn^{2+}\right]\left[2S^{2-}\right]\]
d) \[\left[Sn^{4+}\right]\left[2S^{2-}\right]^{2}\]
Explanation:
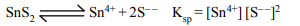
7. How do we differentiate between \[Fe^{3+}\] and \[Cr^{3+}\] in group III?
a) By taking excess of \[NH_4OH\]
b) By increasing \[NH_4^+\] ion concentration
c)By decreasing \[OH^{-}\] ion concentration
d) Both (b) and (c)
Explanation:
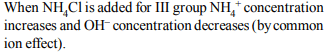
8. The solubility product of barium sulphate is \[1.5 × 10^{-9} \] at 18°C. Its solubility in water at 18°C is
a) \[1.5 × 10^{-9}\] mol \[L^{-1}\]
b) \[1.5 × 10^{-5}\] mol \[L^{-1}\]
c) \[3.9 × 10^{-9}\] mol \[L^{-1}\]
d) \[3.9 × 10^{-5}\] mol \[L^{-1}\]
Explanation:
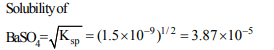
9. The solubility product of AgCl is \[4.0 × 10^{-10} \] at 298 K. The
solubility of AgCl in 0.04 M \[CaCl_{2}\] will be
a) \[2.0 × 10^{-5} M\]
b) \[1.0 × 10^{-4} M\]
c) \[5.0 × 10^{-9} M\]
d) \[2.2 × 10^{-4} M\]
Explanation:
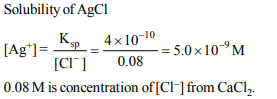
10.The solubility of AgCl at 20°C is\[1.435 × 10^{-3}\] gm/lit. The
solubility product of AgCl is
a) \[1.0 × 10^{-10} \]
b) \[2 × 10^{-10} \]
c) \[1.035 × 10^{-5} \]
d) \[108 × 10^{-3} \]
Explanation: