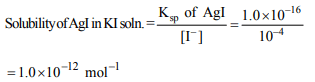1. For preparing a buffer solution of pH 6 by mixing sodium
acetate and acetic acid, the ratio of the concentration of salt
and acid should be \[\left(K_{a} =10^{-5}\right)\]
a) 1 : 10
b) 10 : 1
c) 100 : 1
d) 1 : 100
Explanation:
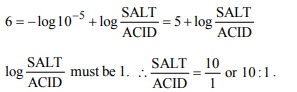
2. What is \[\left[H^{+}\right]\] of a solution that is 0.1 M HCN and 0.2 M
NaCN? \[(K_{a}\] for HCN = \[6.2\times10^{-10})\]
a) \[3.1\times10^{10}\]
b) \[6.2\times10^{5}\]
c) \[6.2\times10^{-10}\]
d) \[3.1\times10^{-10}\]
Explanation:
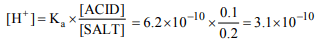
3.The pH of a buffer containing equal molar concentrations
of a weak base and its chloride \[(K_{b}\] for weak base = \[2\times 10^{-5},log 2 = 0.3)\] is
a) 5
b) 9
c) 4.7
d) 9.3
Explanation:
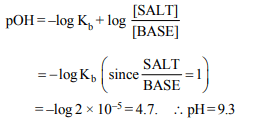
4. How many ml of 1 M \[H_{2}SO_{4}\] is required to neutralise 10 ml of
1 M NaOH solution?
a) 2.5
b) 5.0
c) 10.0
d) 20.0
Explanation:
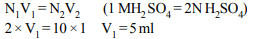
5. Molar heat of neutralization of NaOH with HCl in comparison
to that of KOH with \[HNO_{3}\] is
a) less
b) more
c) equal
d) depends on pressure
Explanation: (NaOH + HCl) (KOH + HNO3 ). Both constitute the pair of strong base and strong acid.
6. The mutual heat of neutralization of 40 g NaOH and 60 g
\[CH_{3}COOH\] will be
a) 57.4 kJ
b) Less than 57.4 kJ
c) More than 57.4 kJ
d) 13.7 kJ
Explanation: 57.4 kJ mol–1 is heat of neutralisation for strong acid and strong base. Since acetic acid is weak, the value will be less than 57.4 kJ mol–1
7. Heat of neutralization of \[NH_{4}OH\] and HCl is
a) equal to 13.7 kcal
b) more than 13.7 kcal
c) less than 13.7 kcal
d) more than one is correct
Explanation: Less than 13.7 kcal. NH4OH is a weak base. It requires less energy for ionisation
8. The pH indicators are
a) salts of strong acids and strong bases
b) salts of weak acids and weak bases
c) either weak acids or weak bases
d) either strong acids or strong bases
Explanation: Weak acids or weak bases e.g. phenolphthalein (weak acid) or methyl orange. (weak base)
9. Identify the indicator used to titrate \[Na_{2}CO_{3}\] solution with
HCl
a) Phenolphthalein
b) \[dil.H_{2}SO_{4}\]
c) Methyl orange
d) None of these
Explanation: Methyl orange is a weak base hence must be used with strong acid
10. The solubility product of AgI at 25°C is \[1.0 × 10^{-16}mol^{2} L^{-2}\] .The solubility of AgI in \[ 10^{-4}N\] solution of KI at 25°C is
approximately (in mol \[L^{-1})\]
a) \[1.0 × 10^{-12}\]
b) \[1.0 × 10^{-10}\]
c) \[1.0 × 10^{-8}\]
d) \[1.0 × 10^{-16}\]
Explanation: